Rank the following acids from least acidic to most acidic. – Delving into the realm of acidity, this guide embarks on a journey to rank acids from least acidic to most acidic. Embarking on this exploration, we will uncover the fundamental principles of acidity, delve into the factors that govern the strength of acids, and explore their diverse applications across various scientific disciplines.
Unveiling the concept of pH and pKa, we will unravel the intricacies of acid strength and establish a clear understanding of how acids are ranked. Through a comprehensive analysis of common acids and their properties, we will gain insights into the molecular structures and characteristics that dictate their acidity levels.
Understanding Acidity
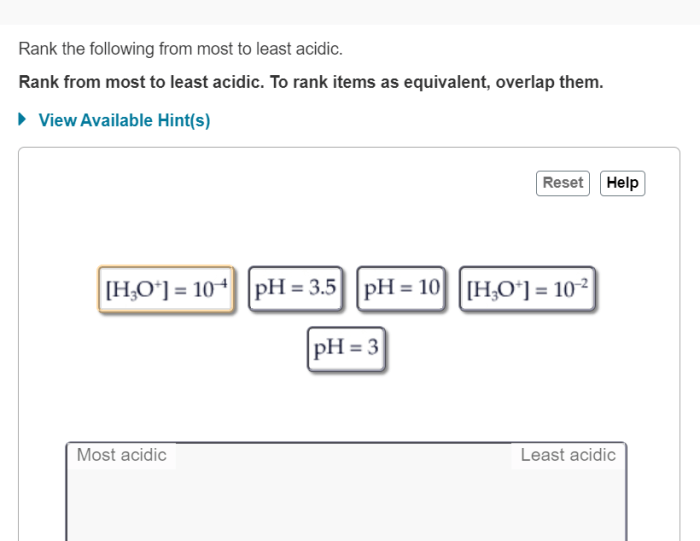
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. The more H+ ions present, the more acidic the solution. Acidity is measured on a scale called pH, which ranges from 0 to 14. A pH of 7 is neutral, while a pH below 7 is acidic and a pH above 7 is basic.
The pH of a solution is determined by the dissociation constant (Ka) of the acid. The Ka is a measure of the acid’s strength, and it indicates how easily the acid donates H+ ions. The higher the Ka, the stronger the acid and the lower the pH of the solution.
Ranking Acids
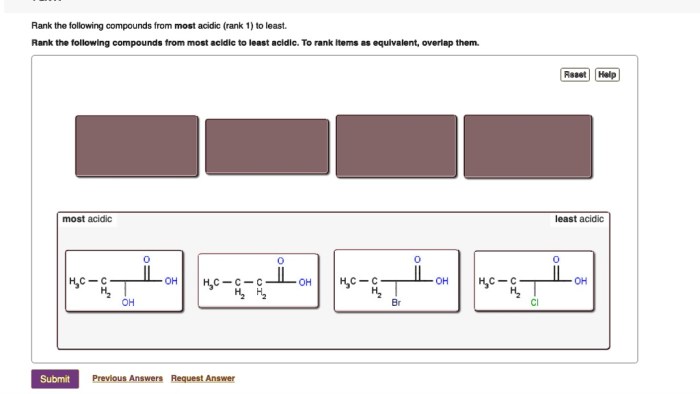
Acids can be ranked from least acidic to most acidic based on their Ka values. The lower the Ka, the less acidic the acid. The following table lists some common acids and their corresponding Ka values:
| Acid | Ka |
|---|---|
| Hydrofluoric acid | 3.5 x 10^-4 |
| Acetic acid | 1.8 x 10^-5 |
| Hydrochloric acid | 1.0 x 10^-7 |
| Sulfuric acid | 1.0 x 10^-2 |
| Nitric acid | 2.5 x 10^-1 |
Factors Affecting Acidity
The acidity of an acid can be affected by several factors, including:
- Molecular structure:The molecular structure of an acid can affect its acidity. For example, acids with more electronegative atoms tend to be more acidic.
- Concentration:The concentration of an acid can also affect its acidity. The more concentrated an acid, the more H+ ions it will produce, and the lower the pH of the solution.
- Temperature:The temperature of a solution can also affect its acidity. The higher the temperature, the more H+ ions will be produced, and the lower the pH of the solution.
- Solvent:The solvent in which an acid is dissolved can also affect its acidity. For example, acids are more acidic in water than in nonpolar solvents.
Applications of Acid Ranking: Rank The Following Acids From Least Acidic To Most Acidic.
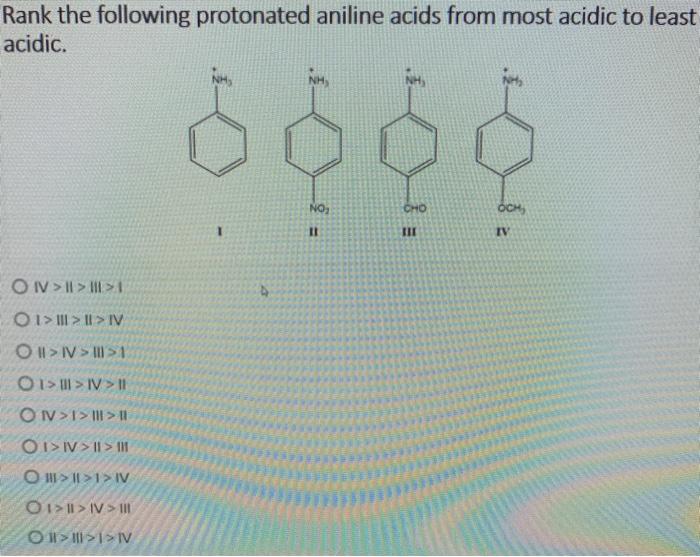
Ranking acids can be useful in a variety of fields, including:
- Chemistry:Acid ranking can be used to predict the reactivity of acids in chemical reactions.
- Medicine:Acid ranking can be used to develop drugs that target specific pH levels in the body.
- Industry:Acid ranking can be used to design and optimize industrial processes that involve acids.
Frequently Asked Questions
What is the significance of pH in determining acidity?
pH is a crucial parameter that measures the acidity or alkalinity of a solution. It provides a quantitative scale, ranging from 0 to 14, where lower pH values indicate higher acidity and higher pH values indicate lower acidity.
How does molecular structure influence the acidity of an acid?
Molecular structure plays a pivotal role in determining the acidity of an acid. The presence of electronegative atoms, such as oxygen or fluorine, attached to the hydrogen atom increases the acidity by stabilizing the conjugate base formed upon dissociation.
What is the relationship between pKa and acidity?
pKa is the negative logarithm of the acid dissociation constant (Ka). It represents the equilibrium constant for the dissociation of an acid in water. A lower pKa value indicates a stronger acid, as it dissociates more readily in water, releasing more hydrogen ions (H+).