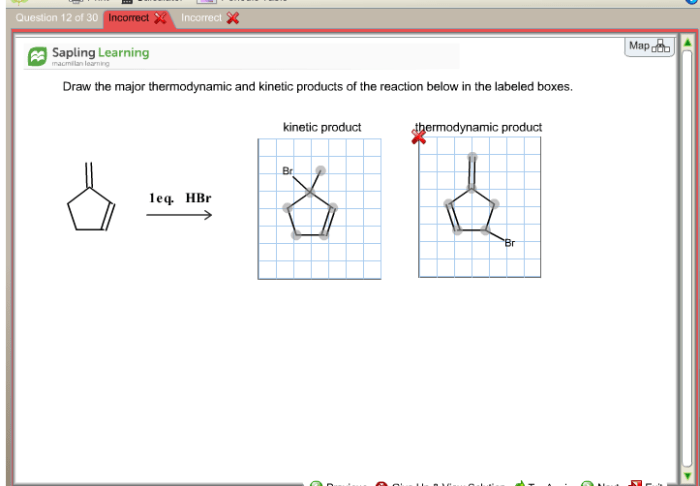
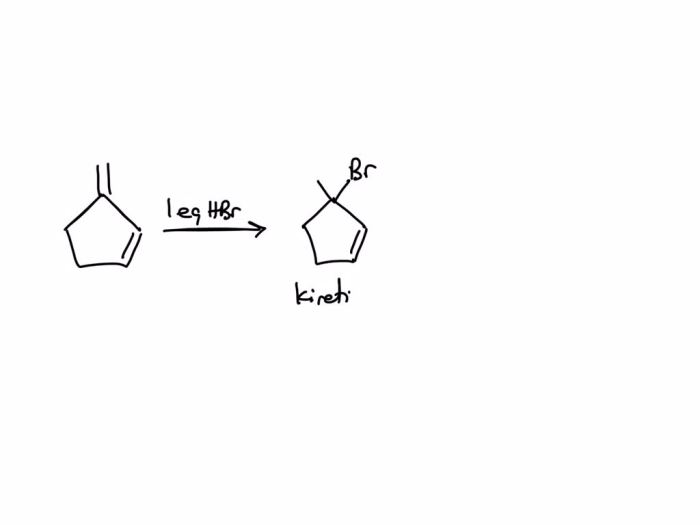
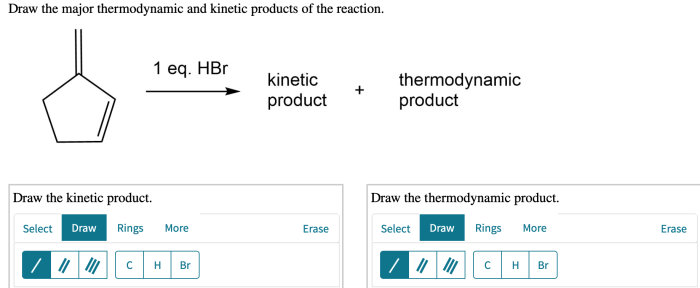
Draw the major thermodynamic and kinetic products of the reaction. – Drawing Major Thermodynamic and Kinetic Products of Reactions, this topic delves into the fascinating realm of chemical reactions, where we explore the factors that govern the formation of products and the methods used to predict their outcomes.
Thermodynamic products are those that are most stable under the given reaction conditions, while kinetic products are those that form more rapidly. Understanding the interplay between these two types of products is crucial for comprehending reaction mechanisms and designing efficient synthetic strategies.
Overview of Thermodynamic and Kinetic Products

In chemical reactions, products can be classified into two types: thermodynamic and kinetic products.
Thermodynamic productsare the most stable products, and they are formed when the reaction reaches equilibrium. Kinetic productsare the products that are formed first, and they are usually less stable than the thermodynamic products.
Factors Affecting Product Formation: Draw The Major Thermodynamic And Kinetic Products Of The Reaction.
The formation of thermodynamic and kinetic products is affected by several factors, including:
- Temperature: The temperature of the reaction can affect the rate of the reaction and the stability of the products.
- Concentration: The concentration of the reactants can affect the rate of the reaction and the equilibrium constant.
- Solvent: The solvent can affect the polarity of the reaction and the stability of the products.
- Catalyst: A catalyst can increase the rate of the reaction without being consumed.
Methods for Predicting Product Formation
Several methods can be used to predict the formation of thermodynamic and kinetic products, including:
- Thermodynamic calculations: These calculations use the free energy change of the reaction to predict the equilibrium constant and the relative stability of the products.
- Kinetic calculations: These calculations use the rate constants of the reaction to predict the rate of formation of the products.
- Experimental methods: These methods involve performing the reaction and analyzing the products to determine their identity and relative abundance.
Applications of Product Formation Prediction
Predicting the formation of thermodynamic and kinetic products has several applications, including:
- Optimization of reactions: By predicting the products of a reaction, chemists can optimize the reaction conditions to maximize the yield of the desired product.
- Design of new materials: By predicting the products of a reaction, chemists can design new materials with specific properties.
- Understanding reaction mechanisms: By predicting the products of a reaction, chemists can gain insights into the reaction mechanism.
FAQ Summary
What are the key factors that influence product formation?
Temperature, concentration, solvent effects, and the presence of catalysts are among the key factors that can influence the formation of thermodynamic and kinetic products.
How can we predict the formation of thermodynamic and kinetic products?
Various methods are available for predicting product formation, including computational modeling, experimental techniques, and the use of thermodynamic tables and kinetic data.